SARS-CoV-2 antigen test kit of Livzon Diagnostics approved for marketing, enabling quick self-testing in 15 minutes
- Time of issue:2022-06-20 18:41
(Summary description)In the evening of April 13, Joincare Pharmaceutical Group Industry Co., Ltd. announced that the Livzon Test for SARS-CoV-2 Antigen (Latex Method) independently developed by Livzon Diagnostics, a company affiliated to its subsidiary Livzon Pharmaceutical Group, was approved by China Food and Drug Administration for marketing. This kit is applicable to nasopharyngeal swabs, oropharyngeal swabs, and nasal swabs. It is easy to use and can provide a clear, easy-to-interpret test result in 15 minutes, without the need for a professional lab. Therefore, the kit can be used for self-testing.
SARS-CoV-2 antigen test kit of Livzon Diagnostics approved for marketing, enabling quick self-testing in 15 minutes
(Summary description)In the evening of April 13, Joincare Pharmaceutical Group Industry Co., Ltd. announced that the Livzon Test for SARS-CoV-2 Antigen (Latex Method) independently developed by Livzon Diagnostics, a company affiliated to its subsidiary Livzon Pharmaceutical Group, was approved by China Food and Drug Administration for marketing. This kit is applicable to nasopharyngeal swabs, oropharyngeal swabs, and nasal swabs. It is easy to use and can provide a clear, easy-to-interpret test result in 15 minutes, without the need for a professional lab. Therefore, the kit can be used for self-testing.
- Time of issue:2022-06-20 18:41
- Views:
In the evening of April 13, Joincare Pharmaceutical Group Industry Co., Ltd. announced that the Livzon Test for SARS-CoV-2 Antigen (Latex Method) independently developed by Livzon Diagnostics, a company affiliated to its subsidiary Livzon Pharmaceutical Group, was approved by China Food and Drug Administration for marketing. This kit is applicable to nasopharyngeal swabs, oropharyngeal swabs, and nasal swabs. It is easy to use and can provide a clear, easy-to-interpret test result in 15 minutes, without the need for a professional lab. Therefore, the kit can be used for self-testing.
As the Omicron variant is spreading rapidly, an easy-to-use and easy-to-popularize test method is needed to identify sources of infections quickly for epidemic prevention and control. Using this test method, large-scale tests can be conducted efficiently. At present, approved antigen test kits in the market use three assay methods: fluorescence immunochromatography method, colloidal gold method, and latex method. The fluorescence immunochromatography method cannot be used for self-testing, because the test results must be interpreted by a specialized device. The other two methods can be used for self-testing, because users can interpret the test results by themselves.
The Livzon Test for SARS-CoV-2 Antigen (Latex Method) has two major advantages over test kits using the colloidal gold method. First, this kit uses colored latex microspheres as markers. Latex microspheres have similar diameters and are 5-10 times larger than common colloidal gold particles. Therefore, the latex method is more sensitive than the colloidal gold method. Second, latex microspheres are mixed with carboxyl and other activating groups when synthesized, and labeled antibodies are covalently linked to latex by chemical bonds. Therefore, the latex method is stabler than the colloidal gold method in which colloidal gold particles and proteins are linked by electric charges.
Specifically, this product is easy to use and allows for quick self-testing without any instrument. Users can know the test result in 15-20 minutes. The product is offered with different specifications to suit different sample types (nasopharyngeal swabs, oropharyngeal swabs, and nasal swabs) and scales of testing (1 test/kit, 5 tests/kit, and 20 tests/kit).
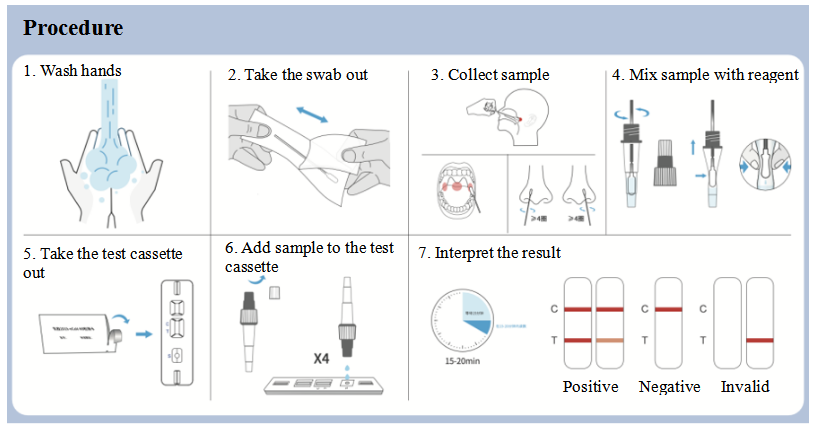
More importantly, clinical trial data of this product is collected from Chinese population. So, it can provide more reliable test results for Chinese people.
In the SARS-CoV-2 Antigen Testing Application Plan released by the National Health Commission of China on March 10, antigen testing was mentioned as a supplement to nucleic acid testing for the first time. Under this circumstance, Livzon Diagnostics accelerated registration of the antigen test kit. The company got all the required documents ready quickly under the guidance of Zhuhai municipal Party committee and government, as well as Xiangzhou district Party committee, government, and food and drug administration. The registration application was submitted to the National Food and Drug Administration on March 29 and approved on April 12 [Product name: Livzon Test for SARS-CoV-2 Antigen (Latex Method); registration number: medical device 20223400470]. Before that, Livzon antigen test kits have been registered in the EU (CE registration), UK, Indonesia, and Thailand, and whitelisted in France, Germany, and Austria. These products have been sold to multiple countries and regions in the EU and Asia-Pacific Region.
The Livzon Test for SARS-CoV-2 Antigen (Latex Method) is intended for in vitro qualitative detection of SARS-CoV-2 N protein antigen. It can be used by healthcare providers or individuals who are under quarantine, have had close contact with COVID-19 patients, or need to perform a self-test. This product can alleviate the pressure of mass testing in China and is of great significance in early detection of SARS-CoV-2 infections.
(This article does not constitute any investment advice. Please check announcements of the company for the information disclosed. Investors who make investment decisions based on this article shall assume the risks arising therefrom.)
Source: National Business Daily
Scan the QR code to read on your phone
Quick Navigation
Service Hotline
Quick
Navigation
Service
Hotline

Official WeChat Official Account
Copyright © Joincare Pharmaceutical Group Industry Co., Ltd. All Rights Reserved. Powered by:www.300.cn Zhuhai 粤ICP备14024104号






