V-01 provides a protective efficacy of 71.83% for at-risk populations, dramatically lowering the risk of severe illness for elderly people and those with underlying medical conditions
- Time of issue:2022-03-28 22:50
(Summary description)The Omicron variant is highly infectious and spreads very quickly. Elderly people with underlying medical conditions or unvaccinated are at higher risk of severe illness after Omicron infection. Experts say that COVID-19 vaccination is particularly important for elderly people in addition to necessary personal protection. Livzon V-01 vaccine is proven to be able to protect this at-risk population effectively: In a phase III heterologous booster trial, V-01 demonstrated a protective efficacy of 71.83% for at-risk populations with underlying medical conditions. Elderly people and those with underlying medical conditions are drawing more attention in the worldwide pandemic prevention and control Most of elder people have underlying medical conditions and are at higher risk of developing severe illness from COVID-19. COVID-19 vaccination can lower the risk of infection and illness, and significantly reduce the probability of severe illness and death. Experts recommend that elderly people who have no contraindications and are eligible for vaccination should be vaccinated as soon as possible. Compared with young people, elderly people generate fewer neutralizing antibodies after vaccinated, and neutralizing antibody titers in their body decrease more significantly over time. Therefore, elderly people are advised to receive a booster six months after the completion of primary vaccination. Currently, several hundred million doses of China-made COVID-19 vaccines have been administrated to elderly people globally, with the highest age of 106 in and outside China. Brazil's Ministry of Health announced on March 23 (local time) that people aged 80 years or older should receive the fourth dose of COVID-19 vaccine four months after the third dose. The Ministry will determine whether people of other age ranges need to receive the fourth dose of COVID-19 vaccine and release an announcement when needed. Livzon Pharmaceutical Group (000513.SZ) announced earlier that it had completed the interim primary data analysis and obtained key data for the phase III heterologous booster trial of the recombinant SARS-CoV-2 fusion protein vaccine (V-01), which was jointly developed by its subsidiary Livzon Mabpharm Inc. and Chinese Academy of Sciences. The Group stressed that heterologous boosting with V-01 provided a high protective efficacy against Omicron. Improving the immunity of at-risk populations is key to pandemic prevention and control, and recombinant protein vaccines may play a crucial role in improving the immunity as boosters. The schedule of Livzon V-01 vaccine covers two shots, and its phase II clinical trial results have been published in the National Medical Journal of China in July 2021. The results show that V-01 has excellent immunogenicity and safety, and the overall incidence of adverse events is lower in the elder group than the younger adult group in comparison. This indicates the potential of V-01 to be used for people of all ages. Brilliant performance of V-01: a protective efficacy of 71.83% for at-risk populations Fudan University published an important study in the BMC Medicine, which compared neutralizing antibody data of several vaccines to assess their long-term neutralizing antibody response kinetics and predict their protective efficacy and immunogenicity. According to the actual efficacy data measured at 28–84 days after heterologous boosting, boosting with Livzon V-01 vaccine after two doses of inactivated vaccines could provide robust protection against Omicron. Clinical trial data proved that Livzon V-01 vaccine could protect at-risk populations effectively: In a phase III heterologous booster trial, V-01 showed a protective efficacy of 71.83% for at-risk populations with underlying medical conditions. Heterologous boosting is an immunization strategy that administrates vaccines developed through different technical approaches at specified intervals and doses to further enhance the protection against viruses. Taking COVID-19 vaccines as an example, heterologous boosting can be achieved with a dose of a recombinant protein vaccine following two doses of inactivated vaccines. V-01 is a new recombinant protein vaccine against COVID-19 jointly developed by Livzon Mabpharm Inc. and Chinese Academy of Sciences since July 2020, with the intellectual property owned by Livzon. Livzon announced that the phase III V-01 heterologous booster trial was the world's first randomized, double-blind phase III clinical trial conducted to study the protective efficacy of heterologous boosting. Compared with phase III trial results of vaccines available in global markets, V-01's absolute efficacy of 61.35% is quite good. This absolute efficacy meets the standard of WHO. According to the announcement of Livzon, the heterologous boosting protocol used in the trial was two doses of inactivated vaccines plus one dose of booster. Unli
V-01 provides a protective efficacy of 71.83% for at-risk populations, dramatically lowering the risk of severe illness for elderly people and those with underlying medical conditions
(Summary description)The Omicron variant is highly infectious and spreads very quickly. Elderly people with underlying medical conditions or unvaccinated are at higher risk of severe illness after Omicron infection. Experts say that COVID-19 vaccination is particularly important for elderly people in addition to necessary personal protection. Livzon V-01 vaccine is proven to be able to protect this at-risk population effectively: In a phase III heterologous booster trial, V-01 demonstrated a protective efficacy of 71.83% for at-risk populations with underlying medical conditions.
Elderly people and those with underlying medical conditions are drawing more attention in the worldwide pandemic prevention and control
Most of elder people have underlying medical conditions and are at higher risk of developing severe illness from COVID-19. COVID-19 vaccination can lower the risk of infection and illness, and significantly reduce the probability of severe illness and death. Experts recommend that elderly people who have no contraindications and are eligible for vaccination should be vaccinated as soon as possible. Compared with young people, elderly people generate fewer neutralizing antibodies after vaccinated, and neutralizing antibody titers in their body decrease more significantly over time. Therefore, elderly people are advised to receive a booster six months after the completion of primary vaccination.
Currently, several hundred million doses of China-made COVID-19 vaccines have been administrated to elderly people globally, with the highest age of 106 in and outside China. Brazil's Ministry of Health announced on March 23 (local time) that people aged 80 years or older should receive the fourth dose of COVID-19 vaccine four months after the third dose. The Ministry will determine whether people of other age ranges need to receive the fourth dose of COVID-19 vaccine and release an announcement when needed.
Livzon Pharmaceutical Group (000513.SZ) announced earlier that it had completed the interim primary data analysis and obtained key data for the phase III heterologous booster trial of the recombinant SARS-CoV-2 fusion protein vaccine (V-01), which was jointly developed by its subsidiary Livzon Mabpharm Inc. and Chinese Academy of Sciences. The Group stressed that heterologous boosting with V-01 provided a high protective efficacy against Omicron.
Improving the immunity of at-risk populations is key to pandemic prevention and control, and recombinant protein vaccines may play a crucial role in improving the immunity as boosters. The schedule of Livzon V-01 vaccine covers two shots, and its phase II clinical trial results have been published in the National Medical Journal of China in July 2021. The results show that V-01 has excellent immunogenicity and safety, and the overall incidence of adverse events is lower in the elder group than the younger adult group in comparison. This indicates the potential of V-01 to be used for people of all ages.
Brilliant performance of V-01: a protective efficacy of 71.83% for at-risk populations
Fudan University published an important study in the BMC Medicine, which compared neutralizing antibody data of several vaccines to assess their long-term neutralizing antibody response kinetics and predict their protective efficacy and immunogenicity. According to the actual efficacy data measured at 28–84 days after heterologous boosting, boosting with Livzon V-01 vaccine after two doses of inactivated vaccines could provide robust protection against Omicron.
Clinical trial data proved that Livzon V-01 vaccine could protect at-risk populations effectively: In a phase III heterologous booster trial, V-01 showed a protective efficacy of 71.83% for at-risk populations with underlying medical conditions.
Heterologous boosting is an immunization strategy that administrates vaccines developed through different technical approaches at specified intervals and doses to further enhance the protection against viruses. Taking COVID-19 vaccines as an example, heterologous boosting can be achieved with a dose of a recombinant protein vaccine following two doses of inactivated vaccines.
V-01 is a new recombinant protein vaccine against COVID-19 jointly developed by Livzon Mabpharm Inc. and Chinese Academy of Sciences since July 2020, with the intellectual property owned by Livzon. Livzon announced that the phase III V-01 heterologous booster trial was the world's first randomized, double-blind phase III clinical trial conducted to study the protective efficacy of heterologous boosting. Compared with phase III trial results of vaccines available in global markets, V-01's absolute efficacy of 61.35% is quite good. This absolute efficacy meets the standard of WHO.
According to the announcement of Livzon, the heterologous boosting protocol used in the trial was two doses of inactivated vaccines plus one dose of booster. Unli
- Time of issue:2022-03-28 22:50
- Views:
The Omicron variant is highly infectious and spreads very quickly. Elderly people with underlying medical conditions or unvaccinated are at higher risk of severe illness after Omicron infection. Experts say that COVID-19 vaccination is particularly important for elderly people in addition to necessary personal protection. Livzon V-01 vaccine is proven to be able to protect this at-risk population effectively: In a phase III heterologous booster trial, V-01 demonstrated a protective efficacy of 71.83% for at-risk populations with underlying medical conditions.
Elderly people and those with underlying medical conditions are drawing more attention in the worldwide pandemic prevention and control
Most of elder people have underlying medical conditions and are at higher risk of developing severe illness from COVID-19. COVID-19 vaccination can lower the risk of infection and illness, and significantly reduce the probability of severe illness and death. Experts recommend that elderly people who have no contraindications and are eligible for vaccination should be vaccinated as soon as possible. Compared with young people, elderly people generate fewer neutralizing antibodies after vaccinated, and neutralizing antibody titers in their body decrease more significantly over time. Therefore, elderly people are advised to receive a booster six months after the completion of primary vaccination.
Currently, several hundred million doses of China-made COVID-19 vaccines have been administrated to elderly people globally, with the highest age of 106 in and outside China. Brazil's Ministry of Health announced on March 23 (local time) that people aged 80 years or older should receive the fourth dose of COVID-19 vaccine four months after the third dose. The Ministry will determine whether people of other age ranges need to receive the fourth dose of COVID-19 vaccine and release an announcement when needed.
Livzon Pharmaceutical Group (000513.SZ) announced earlier that it had completed the interim primary data analysis and obtained key data for the phase III heterologous booster trial of the recombinant SARS-CoV-2 fusion protein vaccine (V-01), which was jointly developed by its subsidiary Livzon Mabpharm Inc. and Chinese Academy of Sciences. The Group stressed that heterologous boosting with V-01 provided a high protective efficacy against Omicron.
Improving the immunity of at-risk populations is key to pandemic prevention and control, and recombinant protein vaccines may play a crucial role in improving the immunity as boosters. The schedule of Livzon V-01 vaccine covers two shots, and its phase II clinical trial results have been published in the National Medical Journal of China in July 2021. The results show that V-01 has excellent immunogenicity and safety, and the overall incidence of adverse events is lower in the elder group than the younger adult group in comparison. This indicates the potential of V-01 to be used for people of all ages.
Brilliant performance of V-01: a protective efficacy of 71.83% for at-risk populations
Fudan University published an important study in the BMC Medicine, which compared neutralizing antibody data of several vaccines to assess their long-term neutralizing antibody response kinetics and predict their protective efficacy and immunogenicity. According to the actual efficacy data measured at 28–84 days after heterologous boosting, boosting with Livzon V-01 vaccine after two doses of inactivated vaccines could provide robust protection against Omicron.
Clinical trial data proved that Livzon V-01 vaccine could protect at-risk populations effectively: In a phase III heterologous booster trial, V-01 showed a protective efficacy of 71.83% for at-risk populations with underlying medical conditions.
Heterologous boosting is an immunization strategy that administrates vaccines developed through different technical approaches at specified intervals and doses to further enhance the protection against viruses. Taking COVID-19 vaccines as an example, heterologous boosting can be achieved with a dose of a recombinant protein vaccine following two doses of inactivated vaccines.
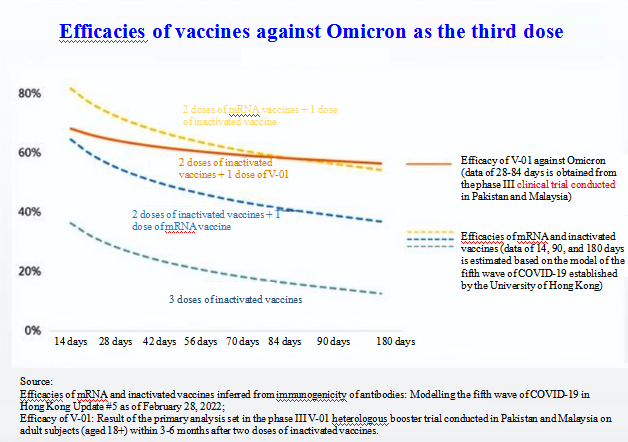
V-01 is a new recombinant protein vaccine against COVID-19 jointly developed by Livzon Mabpharm Inc. and Chinese Academy of Sciences since July 2020, with the intellectual property owned by Livzon. Livzon announced that the phase III V-01 heterologous booster trial was the world's first randomized, double-blind phase III clinical trial conducted to study the protective efficacy of heterologous boosting. Compared with phase III trial results of vaccines available in global markets, V-01's absolute efficacy of 61.35% is quite good. This absolute efficacy meets the standard of WHO.
According to the announcement of Livzon, the heterologous boosting protocol used in the trial was two doses of inactivated vaccines plus one dose of booster. Unlike traditional placebo-controlled trials, this trial measured the relative efficacy of V-01 based on the superiority standard. The phase III V-01 heterologous booster trial conducted in Pakistan and Malaysia planned to administrate one dose of V-01 or placebo for 10,722 adult participants (aged 18 or older) randomly in a 1:1 ratio within 3-6 months after two doses of inactivated vaccines.
As of the date of key data analysis, a total of 10,241 eligible participants received injections, and 110 primary endpoint cases of confirmed COVID-19 were observed (at least 103 primary endpoint cases required for the trial). According to the current trial results, the absolute efficacy of heterologous boosting with V-01 was 61.35%, demonstrating a significant superiority.
Livzon V-01 vaccine elicits robust immunity as a heterologous booster: Results of multiple heterologous booster trials conducted in China and other countries show that V-01 can increase neutralizing antibody titers dramatically in a short time after injection. It provides protection against multiple variants and demonstrates as strong neutralizing activity against Omicron as wild-type strains. The high-titer neutralizing antibodies keep alive for a long time and elicit as high immunity in the elder group as in the younger adult group.
According to preliminary statistics about cluster infections in multiple cities of China in this year, vaccinated elderly people are at lower risk of severe illness than unvaccinated ones. However, COVID-19 vaccination statistics show that, as of February 25, only about 220 million people aged 60 years or older in China have been vaccinated, and about 211 million of them have completed the immunization schedule. The vaccination rate in the elder population, especially people aged 80+years, is still low. Improving the vaccination rate in the elder population is of great significance for reduction of the COVID-19 mortality rate.
Livzon told the press that it was applying for conditional marketing authorization for V-01, and expressed its gratefulness to the support offered by competent departments. There is no doubt that V-01 will offer more choices of booster immunization after it is put on the market, helping to protect people, especially elderly people and those with underlying medical conditions, during the pandemic. We are looking forward to the launch of V-01 to the market.
Source: Weekly on Stocks
Scan the QR code to read on your phone
Quick Navigation
Service Hotline
Quick
Navigation
Service
Hotline

Official WeChat Official Account
Copyright © Joincare Pharmaceutical Group Industry Co., Ltd. All Rights Reserved. Powered by:www.300.cn Zhuhai 粤ICP备14024104号






