Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd.
- Time of issue:2021-01-13 17:08
(Summary description)
Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd.
(Summary description)
- Time of issue:2021-01-13 17:08
- Views:
Founded in September 2013 and located in Zhangjiang Hi-Tech Park, Shanghai, Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd. is the drug research and development center of Joincare Pharmaceutical Group Industry Co., Ltd. in Shanghai. In July 2015, Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd., with the support of the Group, established Guangzhou Joincare Respiratory Drug Engineering Technology Co., Ltd. with the Guangzhou Institute of Respiratory Diseases led by Academician Zhong Nanshan.
Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd. has an excellent R&D team consisting of doctors, masters and undergraduates and other multi-level scientific and technical personnel. It is equipped with more than 140 special equipments with a total value of more than 20 million yuan, including evaluation devices for the effectiveness of various inhalation preparations used in the US and European Pharmacopoeia, respiratory simulators for different populations, dynamic laser diffraction particle size analyzers for inhalation preparations, and other professional analytical instruments such as HPLC.

Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd. focuses on the R&D of therapeutic drugs in the field of major diseases, especially the R&D of inhalation preparations mainly for the treatment of respiratory diseases; drug R&D related pre-prescription research, preclinical pharmaceutical research and project R&D investigation and survey, technical consultation and registration service.

R&D team and project results
The company's R&D team has long been committed to the research of new pharmaceutical dosage forms and their industrialization, involving mangy fields such as inhalation administration, oral sustained and controlled release administration, poorly soluble or thermostable drug injection, and pre-drug prescribing research.
In the past three years since its establishment, the company has been involved in research and development of more than 20 projects including innovative medicines, generic drugs and medical equipment. There are 2 projects that have been submitted for clinical practice, 1 project has received clinical approvals, and 6 projects to be declared. Two new Category I drug R&D projects were funded by the Shanghai Committee of Science and Technology (project numbers: 15431903800 and 16431902500), and one inhalation preparation R&D project was funded by the Science and Technology Commission of Pudong New Area (project number: PKF2015-CO4). In 2014, the company was approved by the Shanghai Committee of Science and Technology to build the “Shanghai Respiratory Drug Delivery Professional Technical Service Platform, project number: 14DZ2293300”, and cooperated with the Shanghai Food and Drug Packaging Materials Testing Institute to jointly establish “Shanghai Drug Compatibility Research Professional Technical Service Platform, project number: 14DZ2293500".
Shanghai Respiratory Drug Delivery Professional Technical Service Platform
Taking the building of “Shanghai Respiratory Drug Delivery Technical Service Platform” as an opportunity, Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd. has established a research service platform for research, evaluation and engineering development of respiratory drugs to specialize in the development of respiratory drug varieties and the research on key industrialization engineering techniques, accelerate the transformation of technological achievements and industrial technology upgrades through breaking through the bottlenecks in variety design and localization, and provide CRO services of preparation research and related technical consultation for pharmaceutical companies and research institutions at home and abroad.
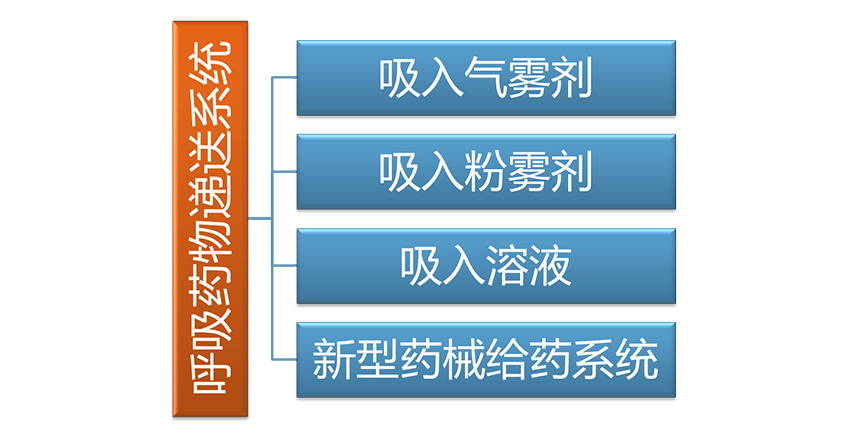
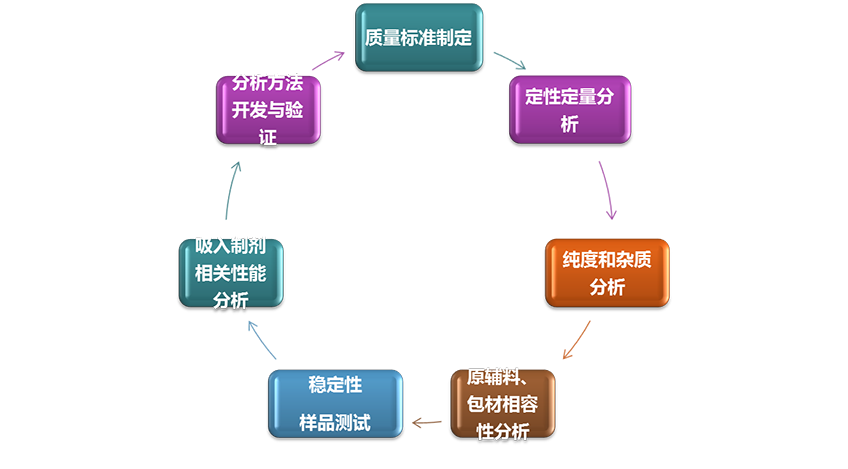
Third party testing service
Combined with the respiratory drug delivery system R&D, characterized by inhalation preparation related testing and quality evaluation, the company actively prepares a third-party pharmaceutical testing service platform to conduct drug R&D related quality research and testing. An application for testing laboratory accreditation (CNAS) has been submitted to the Certification and Accreditation Administration of the People’s Republic of China.
Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd. sincerely welcomes domestic and foreign pharmaceutical companies, universities and scientific research institutions to cooperate and exchange.
Contact information:
Building 1, No. 899, Zu Chongzhi Road, Pudong New Area, Shanghai
Contact: Mr. Wen
Contact number: 021-58368016
Fax: 021-58368002
Scan the QR code to read on your phone
Quick Navigation
Service Hotline
Quick
Navigation
Service
Hotline

Official WeChat Official Account
Copyright © Joincare Pharmaceutical Group Industry Co., Ltd. All Rights Reserved. Powered by:www.300.cn Zhuhai 粤ICP备14024104号






