The first domestically approved
Published Time:
2020-02-20
On September 27, Health Yuan's subsidiary, Shenzhen Tai Tai Pharmaceutical, obtained approval from the National Medical Products Administration for its levalbuterol hydrochloride nebulization solution, submitted under the new Class 3 registration, becoming the first levalbuterol preparation (trade name: Lishutong) to be marketed in China.
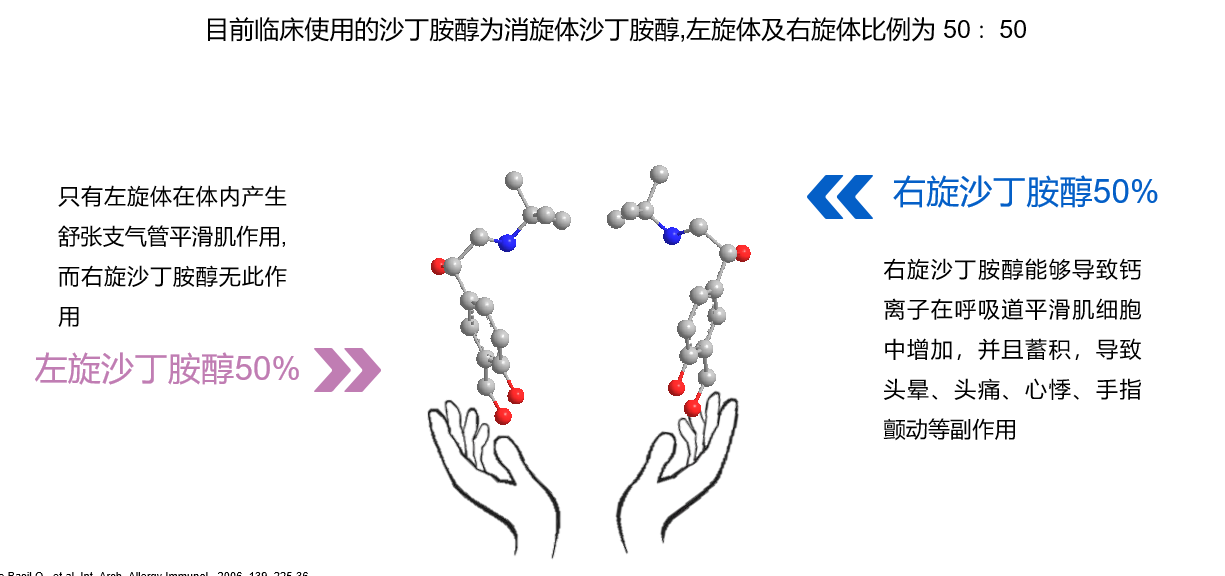
Currently in China, the incidence of respiratory diseases such as asthma and chronic obstructive pulmonary disease continues to increase, with patients accounting for as much as one-tenth of the total population. However, due to a general lack of patient awareness, the diagnosis and standardized treatment rates remain severely low. Nebulization therapy is currently a commonly used method of drug administration for patients with respiratory diseases. However, according to IMS data, in the Chinese market for nebulized inhalation drugs in 2018, 91% was occupied by foreign pharmaceutical companies. Due to the high R&D threshold in the field of nebulized inhalation preparations, there are almost no better domestic alternatives.
Health Yuan's newly approved levalbuterol hydrochloride nebulization solution (Lishutong) is expected to break the deadlock of the nebulized inhalation drug market being highly monopolized by foreign pharmaceutical companies, and also provides domestic doctors with more choices in the field of respiratory disease medication. It is worth noting that on June 6, 2018, the list of drug registration applications included in the priority review procedure by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) (29th batch) included 3 pediatric drugs, and Health Yuan's levalbuterol hydrochloride nebulization solution was among them. From the inclusion in the priority review procedure application, it took the NMPA only one year and two months to approve Lishutong for marketing, indicating the urgent market demand for this drug and highlighting the profound technical R&D background of Health Yuan's R&D team.
91% of inhalation preparations are monopolized by foreign companies
A research report from Ping An Securities in June of this year stated that chronic respiratory diseases are the third leading cause of death from chronic diseases among Chinese residents: China's chronic disease mortality rate reached 533/100,000 in 2012, with chronic disease deaths accounting for 86.6%, of which the mortality rate for chronic respiratory diseases was 68/100,000, accounting for 12.8%, making it the third leading cause of death from chronic diseases after cardiovascular and cerebrovascular diseases and tumors.
According to statistics from the Ministry of Health, the incidence of respiratory diseases in China accounts for about 6.94% of the total incidence, and 150 million people suffer from various respiratory diseases every year.
Faced with a patient population of over 100 million, there are few treatment methods, and the treatment drugs are not only limited in variety but are also controlled by foreign pharmaceutical companies. Asthma, as a chronic inflammatory disease, can be controlled through long-term use of corticosteroids, while the treatment of acute exacerbations of asthma and COPD is centered on bronchodilators, mainly to relieve airway spasms and improve ventilation, with some overlap in medication. In terms of dosage forms, inhalation dosage forms have gradually become mainstream. According to research reports, the global market for asthma and COPD drugs exceeded US\$45 billion in 2017, with the inhalation preparation market reaching US\$37 billion. Blockbuster products have frequently emerged in this field, with the average sales of the top 10 products in the United States in 2017 being around US\$2 billion.
The report also analyzes that the current domestic market for asthma and COPD drugs is approximately 17 billion yuan, with a CAGR of 14% from 2012 to 2018, of which foreign companies account for 78%, and inhalation dosage forms account for 67%. Among inhalation dosage forms, foreign companies account for 93%, with AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline accounting for 91% of the market.
For example, Seretide is GSK's best-selling inhaled asthma or COPD drug, but it lost its US patent protection in 2010. Even years after the patent expired, it has not been affected by generic drugs. The main reason is that Seretide is a combination product of a drug and an inhaler. The production process of this combination product is relatively complex, and it is more challenging to develop than solid oral dosage forms such as tablets. The US FDA only approved the first generic drug for Seretide in February of this year.
The NMPA has indeed approved quite a few inhalation preparations before, but they were all approved before the implementation of the new chemical drug registration classification. According to information from the Pharmaceutical Cube database, between 2016 and 2018, before Health Yuan's Shutanlin (inhalation compound ipratropium bromide solution) was approved, the NMPA only approved 6 inhalation preparations for the first time, and all of them were registered under the old Class 6 system. Four of them were inhalation acetylcysteine solutions for expectoration, which still require re-evaluation for consistency after approval (see: Health Yuan's "inhalation compound ipratropium bromide solution" becomes the first generic drug to be marketed, ushering in a major change in the domestic inhalation preparation market ).
Lishutong ® Accelerating the domestic substitution of inhalation preparations
Source: Pharmaceutical Cube



