-
Blog BLOG
-
Product Center BRAND DYNAMICS
-
Investor INVESTOR RELATIONS
-
Contact Us CONTACT
-
Supervise SUPERVISION
The generic name "Mapasivir" for Jiankangyuan's innovative influenza drug has been approved, bringing its market launch closer.
Published Time:
2024-12-05
Recently, Jiankangyuan received notification from the National Pharmacopoeia Commission that its innovative influenza drug Generic Name has been approved for registration and is officially named " Mapaxiwa " (the Chinese generic name for the raw material is "Mapaxiwa", and the English name is "Pixavir Marboxil"; the Chinese generic name for the preparation is "Mapaxiwa Capsules", and the English name is "Pixavir Marboxil Capsules").
Mapaxiwa is an anti-influenza Class 1 innovative drug, a novel cap-dependent endonuclease inhibitor that effectively blocks viral replication and transmission, with characteristics of rapid onset, long viral suppression time, good tolerability, and oral administration unaffected by food It can effectively inhibit both influenza A and B viruses . Compared to oseltamivir, the mainstream influenza drug currently on the market, Mapaxiwa has a long-acting advantage, requiring only one dose for the entire course of treatment.
01
Successful Phase III Clinical Trials, Excellent Data
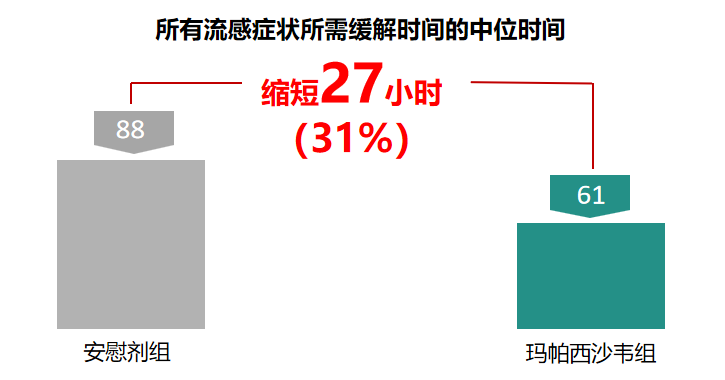
According to the company's announcement, in April of this year, the Phase III clinical trial of this drug reached its primary endpoint, and the results confirmed that the median time to relief of all influenza symptoms was superior to that of the placebo group. The application for the registration and listing of domestically produced drugs was accepted by the drug administration in August this year.
Based on a multicenter, randomized, double-blind study of 752 cases in Phase 3 clinical trials. The median time to relief of all influenza symptoms within 15 days of the treatment period in the TG-1000 group and the placebo group was 60.9 hours and 87.9 hours, respectively, showing that the primary efficacy endpoint was reached and there was a statistically significant difference (P<0.0001).
02
Comparison Advantages with Existing Influenza Treatment Regimens
Meanwhile, compared with oseltamivir and baloxavir marboxil, the mainstream anti-influenza drugs currently on the market, Mapaxiwa has shown the following advantages in clinical practice:
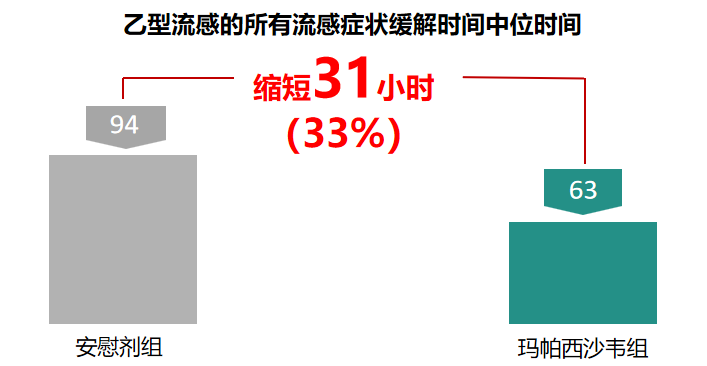
1、 Faster Relief of Influenza B
Compared with baloxavir marboxil and oseltamivir, TG-1000 can significantly reduce the time to relief of influenza B symptoms and effectively control influenza B.
Clinical Phase III median time to relief of all influenza symptoms in influenza B:
Mapaxiwa shortened by 31h (63.3h vs 94.3h) compared to placebo, with a statistically significant difference (p<0.05).
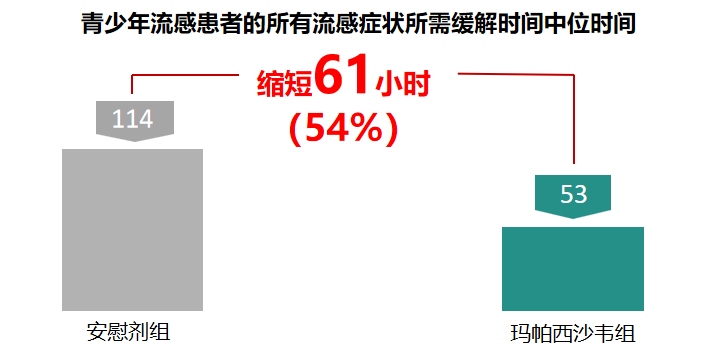
2、 Significant Benefits for Adolescent Patients
In Phase III clinical studies, Mapaxiwa showed faster symptom relief in adolescent influenza patients, as well as better therapeutic efficacy and safety. With the subsequent launch of this new drug, we expect it to bring more health and peace of mind to adolescents during the influenza season.
Clinical Phase III median time to relief of all influenza symptoms in adolescent influenza patients:
Mapaxiwa shortened by 61.0h (53.0h vs 114.0h), with a statistically significant difference (p<0.01).
3、 Single Dose Administration
Compared with oseltamivir, which needs to be taken twice a day, Mapaxiwa has a long viral suppression time and only needs a single oral dose. A dose of 40mg or 80mg is sufficient to achieve the therapeutic effect, significantly improving clinical compliance, simplifying the treatment process, and having excellent clinical value.
In addition, drug resistance is a major challenge for anti-influenza drugs, while Mapaxiwa has lower drug resistance and is not affected by food intake. This means that patients will be able to obtain a more convenient medication experience with less drug failure.
03
Market Launch Expected Respiratory Leader Achieves Self-Innovation Breakthrough
The anti-influenza drug "Mapaxiwa", with its advantages of only requiring one dose for the entire course of treatment, rapid onset, and simultaneous action against both influenza A and B, is expected to stand out in the tens of billions of anti-influenza drug market, providing patients with more and better treatment options. The approval of Jiankangyuan's name means that this innovative drug is one step closer to market launch.
Currently, Jiankangyuan is working hard to complete the pre-market work. If it is successfully launched in the future, we believe that the influenza season will no longer be a worrying time, but a time that can be effectively managed with new scientific achievements.
Quick Navigation
+86 755-33268688 | Service Hotline

Official WeChat Official Account
Copyright © 2025 Joincare Pharmaceutical Group Industry Co., Ltd. ALL Rights Reserve.
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Sorry,当前栏目暂无内容!
您可以查看其他栏目或返回 首页
Sorry,The current column has no content!
You can view other columns or return Home


