Shenzhen Tai Tai Gene Engineering Co., Ltd.
Company Profile
Shenzhen Tai Tai Gene Engineering Co., Ltd. was established in 2002 with a registered capital of 50 million RMB. The company is a wholly-owned subsidiary of Health Yuan Pharmaceutical Group (Shanghai Stock Exchange Code: 600380). It mainly engages in the research and development, production, sales, and technical services of gene diagnostic reagents and related products. The company has over 800 square meters of cleanroom, PCR testing equipment, and nucleic acid automated extraction workstations. Product areas include food safety, prevention and control of major human infectious diseases, prevention and control of animal diseases, and entry-exit inspection and quarantine.
Company Honors
Recognized as a national high-tech enterprise in 2008.
Recognized as a Shenzhen high-tech enterprise in 2014.
Since 2008, it has been jointly recognized by the Shenzhen Municipal Bureau of Commerce, Shenzhen Municipal Environmental Protection Bureau, and Shenzhen Municipal Bureau of Science and Technology and Information as
a "Clean Production Enterprise".
Products and Achievements
As a technology-innovation-oriented national high-tech enterprise, the core business is the research, development, and industrialization of new technologies, new processes, and new products. It has established an internationally leading real-time quantitative PCR technology platform and has undertaken more than 10 scientific research projects from the Shenzhen Municipal Bureau of Science and Technology and Information, Nanshan District Bureau of Science and Technology and Information, Dongguan Municipal Bureau of Science and Technology, and Guangzhou Municipal Health Bureau. Among them, the "Development of Avian Influenza H5, H7, H9 subtype real-time fluorescence RT-PCR detection reagents" in cooperation with the Shenzhen Entry-Exit Inspection and Quarantine Bureau won the first prize of the "Science and Technology Advancement Award" of the State Administration for Market Regulation in 2004, and the "Development of fluorescence PCR detection kits for Listeria monocytogenes, Salmonella, and Staphylococcus aureus" in cooperation with the Dongguan Entry-Exit Inspection and Quarantine Bureau won the first prize of the Dongguan Municipal Bureau of Science and Technology. From 2004 to the present, a total of more than 4 million yuan in government-sponsored scientific research funds have been received.
The company's research and development achievements have applied for and obtained 12 patents, participated in the formulation of three Chinese entry-exit inspection and quarantine industry standards, and developed 42 real-time quantitative PCR detection kits. Among them, the "Foodborne Pathogenic Microorganism Fluorescence PCR Detection Kit" and the "Avian Influenza Virus Fluorescence PCR Detection Kit" were awarded the Shenzhen Municipal Bureau of Science and Technology and Information's independent innovation product certification in 2008 and are widely used in entry-exit inspection and quarantine systems, CDC systems, and animal disease prevention and control systems.
Invention Patent Catalog
| Name |
Application No./Patent No. |
| A kit for detecting Helicobacter pylori infection |
201120390768.6 |
| A kit for detecting Helicobacter pylori infection |
201120390807.2 |
| A follicle-stimulating hormone colloidal gold test card |
201120390790.0 |
| A primer pair, probe, and kit containing them for detecting avian influenza virus in samples by fluorescence RT-PCR |
201310216547.0 |
| A primer pair, probe, and kit containing them for detecting avian influenza virus in samples by fluorescence RT-PCR |
201310216098.X |
| A primer pair, probe, and kit containing them for detecting avian influenza virus in samples by fluorescence RT-PCR |
201310216523.5 |
| A primer pair, probe, and kit containing them for detecting avian influenza virus in samples by fluorescence RT-PCR |
201310216539.6 |
| Primer and probe sequences for detecting Vibrio parahaemolyticus nucleotide fragments |
200510120893.4 |
| Primer and probe sequences for detecting Shigella nucleotide fragments |
200510120894.9 |
| Primer and probe sequences for detecting Vibrio cholerae O1 nucleotide fragments |
200510120895.3 |
| Primer and probe sequences for detecting Vibrio cholerae nucleotide fragments |
200510120897.2 |
| Primer and probe sequences for detecting Vibrio cholerae O139 nucleotide fragments |
200510120896.8 |

National High-tech Enterprise Certificate Shenzhen High-tech Enterprise Certificate




Shenzhen Independent Innovation Product Certification




Clean Production Enterprise Certificate Nucleic Acid Workstation


Invention Patent Certificates




Industry Standards for Entry-Exit Inspection and Quarantine formulated with the participation of the company
SN/T 1870-2007 Detection Method for Pathogenic Bacteria in Food - Real-time PCR Method
Issued by the State Administration for Market Regulation on April 6, 2007, implemented on October 16, 2007
This standard specifies the real-time PCR detection methods for Salmonella, Shigella, Staphylococcus aureus, Yersinia enterocolitica, Listeria monocytogenes, Campylobacter jejuni, enterohemorrhagic Escherichia coli O157:H7, Vibrio parahaemolyticus, Vibrio cholerae, Vibrio vulnificus, and Vibrio alginolyticus in food. SN/T 1869-2007 Rapid Detection Method for Multiple Pathogenic Bacteria in Food - PCR Method
Issued by the State Administration for Market Regulation on April 6, 2007, implemented on October 16, 2007
SN/T 1632.3 Test Method for Cronobacter sakazakii in Infant Formula - Part 3: Fluorescence PCR Method
Issued by the State Administration for Market Regulation on August 18, 2005, implemented on February 1, 2006
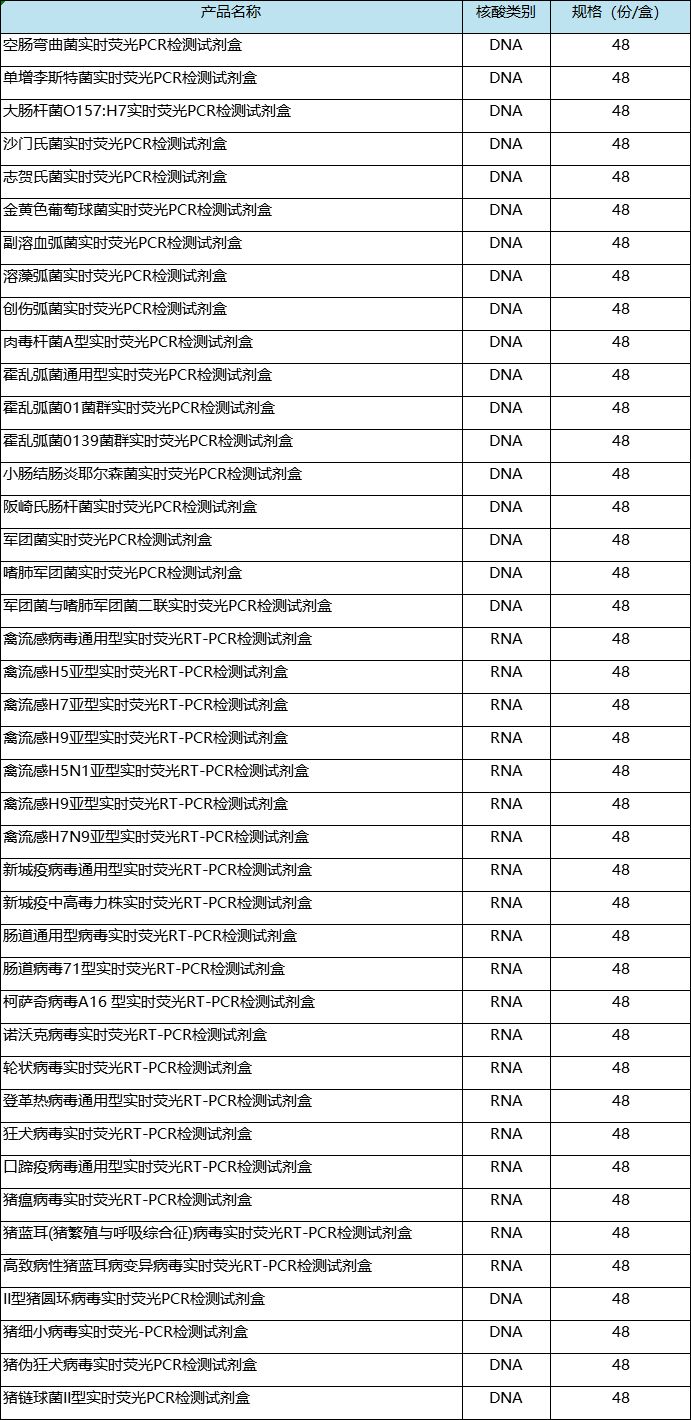
Product Catalog


Contact Information
Tel: 0755-26994502
Address: 3rd Floor, Tai Tai Pharmaceutical Industrial Building, 5th Industrial Zone, Nanshan District, Shenzhen
地址:深圳市南山区第五工业区太太药业工业大厦三楼



